[ad_1]
Last Updated on December 15, 2022 by GlobeNewsWire
SAN DIEGO, Dec. 15, 2022 (GLOBE NEWSWIRE) — Biosplice Therapeutics, Inc. (“Biosplice”), a clinical-stage biotechnology company pioneering first-in-class therapeutics based on small-molecule inhibition of CLK/DYRK kinases, announced today potential structure-modifying highlights from an ongoing long-term extension study, OA-07, for its knee OA drug candidate, lorecivivint (“LOR”). Results from the study continue to support the potential efficacy of multiple injections of lorecivivint in delaying structural progression and providing symptomatic benefic.
“We believe these unique data highlight lorecivivint’s promise to potentially provide structural benefit to the millions of patients suffering from osteoarthritis,” commented Biosplice Chief Medical Officer, Yusuf Yazici, MD. “These latest interim results from OA-07 build upon our robust body of existing evidence, suggesting that repeat injections of LOR could provide patients and physicians with both structure-modifying and symptomatic benefits. We recently initiated our phase 3 OA-21 clinical trial and are enrolling subjects. We are excited to continue to investigate LOR as a treatment option for knee OA.”
OA-07 is a long-term extension trial, which enrolled patients who completed OA-11, a previous 12-month study. The primary efficacy objective of OA-07 is to measure the disease-modifying potential of lorecivivint over multiple years and injections. During the first year of the OA-07 study, patients and investigators remained blinded and patients received injections of LOR on an annual basis, allowing Biosplice to capture both long-term and repeat-injection data for safety, efficacy and mJSW.
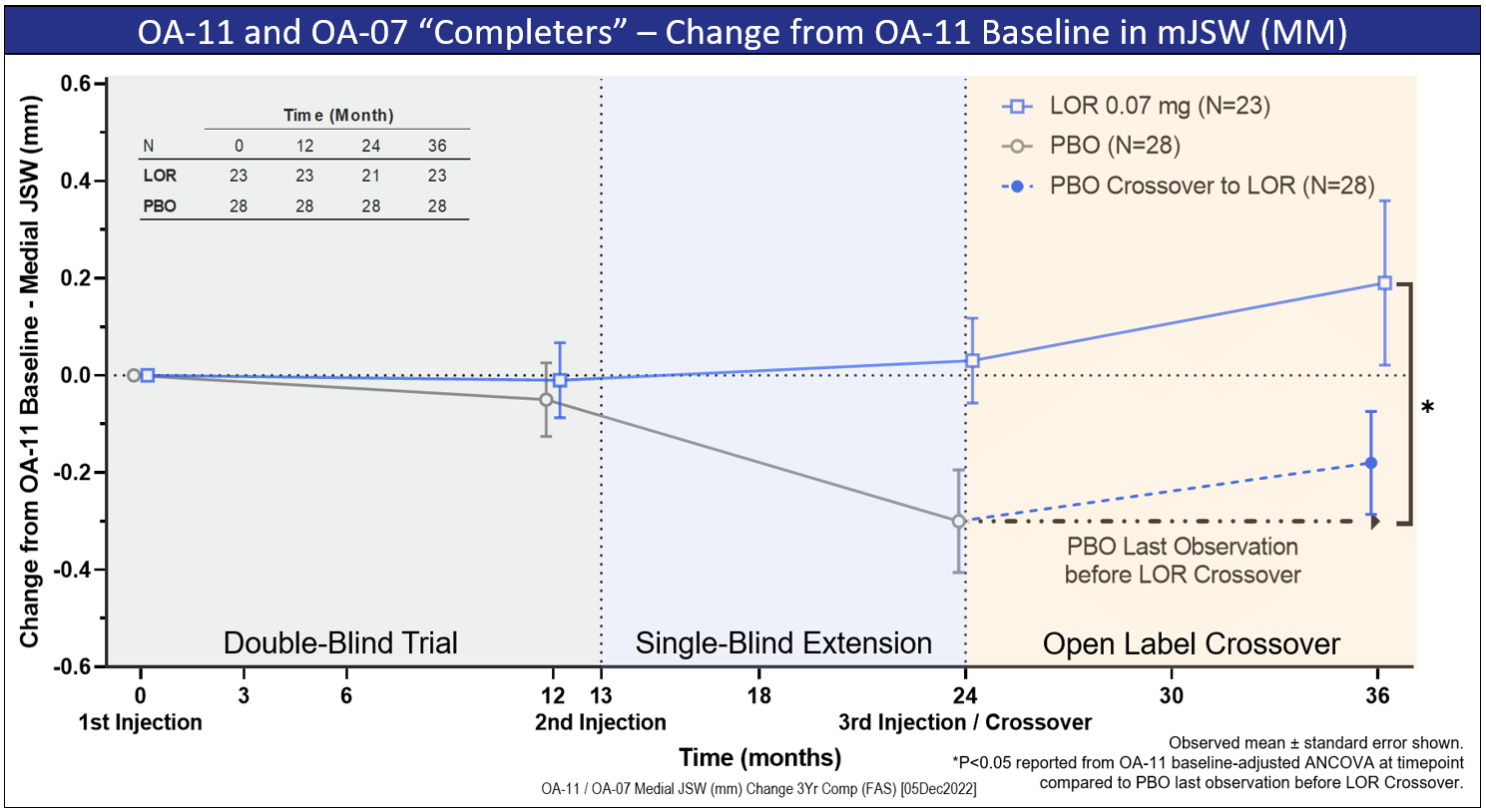
Previously presented at the American Conference of Rheumatology Annual Meeting in November 2022, data for the first year of OA-07 show clinically significant improvement in mJSW relative to placebo, with an effect size of 0.13 mm in KL2 subjects. Moreover, OA-07 data demonstrate a significant improvement in WOMAC Pain and robust trends in Pain NRS (daily pain score) as well as WOMAC function in all subjects.
In the second year of the OA-07 trial, placebo subjects crossed-over to receiving LOR, although all subjects remain blinded as to whether they had previously received LOR or placebo. In subjects who have completed their second annual visit and who previously received LOR in OA-11 and OA-07, there continues to be an increase in their mJSW versus baseline. These subjects have now received three injections of LOR over three years.
Among those subjects who have completed their second annual OA-07 visit and who previously had received placebo, the introduction of LOR appears to have arrested their decline in mJSW.
Please see graphic below for the interim data generated to date.
A graphic accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/e78b4ca6-1a11-47d3-af52-d01735ca5776
Consistent with the clinical program to date, LOR continued to appear safe and well-tolerated in the recently completed studies, with no safety signals with repeat injections.
The OA-21 phase 3 clinical trial is designed as a 16-week study with a primary endpoint of Pain NRS at 12 weeks. This study will further evaluate the impact of LOR on knee osteoarthritis pain and function and has been designed to enroll the less structurally damaged patient population that has shown consistent responses across Biosplice’s phase 2 and phase 3 clinical trials.
About BiospliceBiosplice is developing first-in-class, small-molecule therapeutics based on pioneering science of alternative pre-mRNA splicing. Stemming from foundational discoveries in Wnt pathway modulation, Biosplice has elucidated novel biology linking CLK/DYRK kinases to the therapeutic regulation of alternative splicing. Alternative splicing is an essential biological mechanism that regulates the diversification of proteins in a cell, which, in turn, determines cell type and function. Biosplice’s target class governs the selection of tissue-specific mRNA splice sites, making them attractive, druggable targets within the cellular “command and control” center.
Biosplice’s drugs in clinical development include lorecivivint for osteoarthritis (in phase 3), cirtuvivint for numerous cancers, and a broad pipeline that ranges from Alzheimer’s disease to other degenerative conditions. Learn more at https://www.biosplice.com
[ad_2]
Image and article originally from biotechwinners.com. Read the original article here.